Atomic Radius Across a Period
The reason is that youre also adding more protons which exert a stronger attractive force on the electrons drawing them in a tiny bit closer. 252879C 423182F 20271 K Block.
How Does Atomic Radius Change As You Move Across The Periodic Table Quora
Covalent radius Half of the distance between two atoms within a single covalent bond.
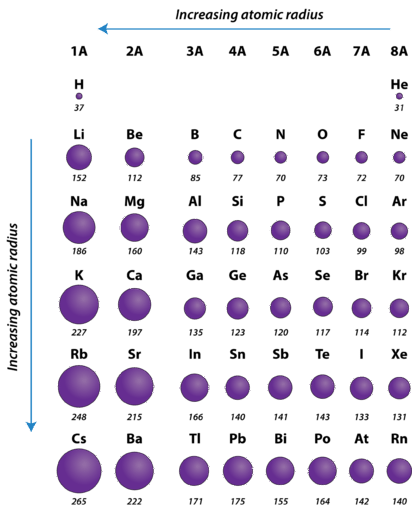
. This is because each atom further down the column has more protons and neutrons and also gains an additional electron energy shell. Thus the increasing number of nucleus attracts the more electrons more tightly towards it and the atomic radius decreases. These values were determined using several different methods.
Atomic radius decreases across the period. Atomic radius increases down the group. Even though each atom has more electrons as you move from left to right across the periodic table the atomic radius decreases.
Period 1 Boiling point. Atomic radius is one of the periodic properties of the elements. As you move down an element group column the size of atoms increases.
Ionic radius also decreases although not for the exact same reason. Atomic radius non-bonded Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. If you look at the table you can see there is a clear trend in atomic radius.
Moving from left to right across a period the number of protons and electrons increases while the number of energy shells stay same.
What Is The Trend In Atomic Radius As You Go Across A Period Socratic
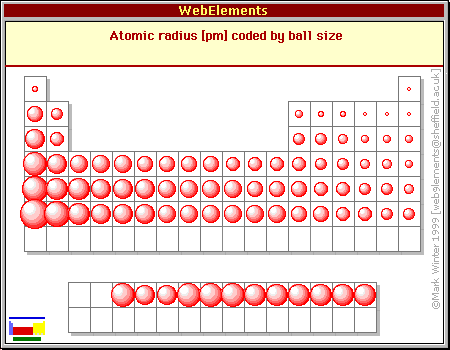
How Does Atomic Radius Change From Left To Right Across A Period In The Periodic Table Socratic

Chemistry Periodic Variations 7 Of 23 Atomic Radius What Determines The Radius 3rd Period Youtube

Why Does Atomic Size Decrease As You Move From Left To Right Across A Period Quora
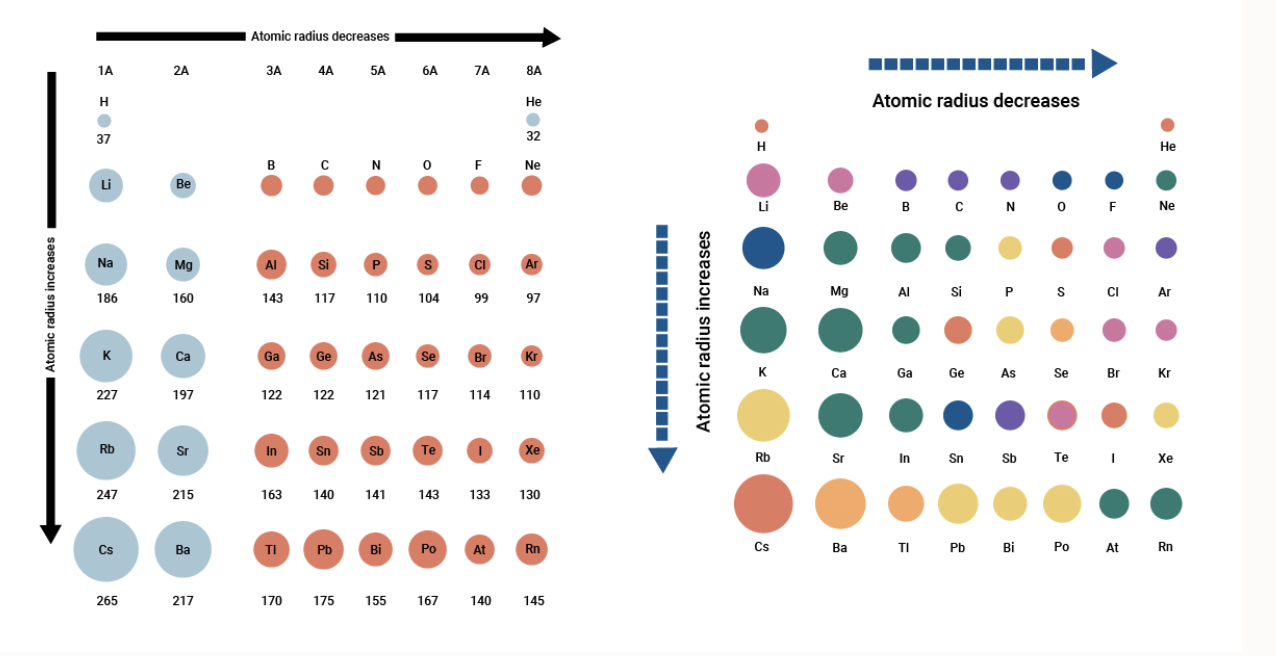
Comments
Post a Comment